High quality engineering is made possible only by constantly testing the quality of the equipment. In piping, it is made sure that the integrity of the component has not been compromised. For the purpose of inspecting pipes and evaluating distribution of corrosion around the circumference, long range ultrasonic testing is one of the most preferred testing methods, also known as LRUT. This method is frequently used to inspect long pieces of pipes and proves especially valuable where the location is inaccessible such as culvert or those at high elevation, making it an economical way to test the pipes without spending on excavation, insulation removal and scaffolding.
IRC Engineering Services provides in-depth services that employ LRUT for accurate assessment of the condition of piping. Our inspectors are thoroughly qualified to assess piping through long range ultrasonic testing helping to maintain the best condition through early detection of any possible corrosion or ruptures.
Method
LRUT is performed through a process utilising low frequency flaw detector, pulse receiver, transducer rings and a monitoring apparatus such as a computer or laptop. The transducer rings are placed around the pipe and a low frequency set of waves is then generated, which traverses along the length of the pipe. When placed at equal distance from each other, these transducers emit waves that move symmetrically through the length of the pipe. Therefore, once these waves meet the corroded portions of the pipe, they are reflected back to the transducer.
Since this method does not destroy the piping in any way and reduces the need to excavate the entire pipe to check for flaws, it is a cost effective method of testing that is preferred by industries all over the world. Our equipment Teletest FOCUS uses optimum level of ultrasound that is required to prime this equipment to be most sensitive to even the most minimal damage to piping. One can therefore rest assured that piping can be checked for all damages.
Benefits
- In-service inspection prevents production losses or downtime.
- Entire length of the pipe can be assessed for integrity at a one time.
- Examine 180 meters from a single test location. (90m on each side of transducer)
- Examine 100% of the pipe circumferential wall from a single test location.
- Reduction in maintenance cost as there is no requirement of removal of surface coating or insulation except the location at which the transducer has to be fixed.
- C-scan imaging gives a pictorial view of the scanned area which helps in locating the defects.
- GPS system installed in the equipment helps to locate inspected area.
- Secondary focusing technique is a unique feature to focus the concerned area.
- No couplant is required for the transmission of ultrasound.
Applications
LRUT has several field applications in various industries. The most popular use of LRUT is in the detection of corrosion. Full set of applications for long range ultrasonic testing is mentioned below:
- Assessing pipes that are located deep below the surface of the ground.
- Assessing pipes that are deep within walls or are encased.
- Assessing pipes that are otherwise inaccessible such as overhead pipes, etc.
- Assessing insulated pipes, as this method can detect the sound waves even through surface coating or padding.
- Can be used to detect corrosion, areas of concern, weld root erosion for piping in refineries, chemical plants, power stations, underwater, in farms, sewers, etc.
IRC Engineering Services recommends that LRUT be performed in conjunction with other testing methods such as phased array ultrasonic testing, which will additionally test the pipe to check for the precise thickness of the pipe wall. Pulsed Eddy Current testing can also be done to check for the average thickness of the pipe wall, in case only a general figure is required.
Through this method of nondestructive testing, industries can detect any concerns with their piping to prevent or correct adverse situations such as pipe corrosion. With the help of our highly qualified engineers, you will receive a full assessment of the condition of your pipes through our precision methodology.
Limitations
While long range ultrasonic testing of piping is an effective method to gauge any damage in piping, this method does have certain limitations. It is important to be aware of these limitations so that the correct tests can be performed on the piping to do a thorough check for corrosion, leakage etc.
- Gives only an approximate measurement of wall thickness: Long range ultrasonic testing can detect variations in thickness of pipe walls, but does not provide an accurate estimation of the wall thickness. For this, a combination of tests can be done, included PAUT, PECT together with LRUT.
- Complexity in assessment of pipes in waterlogged conditions: In case the pipe is lying beneath the surface in wet ground, travelling of sound waves can be made more difficult. This can hamper the testing process, leading to inaccurate results. It is therefore prescribed for use when the pipe is lying in dry conditions.
- Cannot be used in very narrow or short pipes: Due to the passage of sound waves, the pipe has to have a minimum diameter of 1.5 inches in order for proper testing to be done. The pipe also needs to be of a suitable length to accurately assess. The recommended minimum pipe length is 5 meters.
- Does not differentiate between type and location of corrosion: Long range ultrasonic testing is an effective method to detect the presence of corrosion, but does not allow the assessment of corrosion that is taking place actively or passively. It also does not allow the assessor to determine if the corrosion is taking place within the pipe or on its surface. Further testing using other methods needs to be done in this case.
Long range ultrasonic testing of pipes is meant to be used primarily as a screening method for pipe damage and should not be used to determine specifics about the type of corrosion, exact wall thickness etc.
read more IRC Engineering Services
IRC Engineering Services
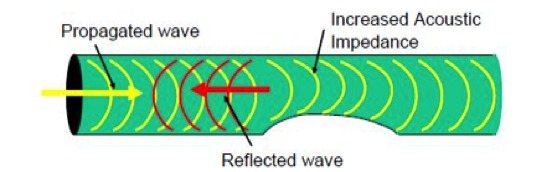




 Photo 1
Photo 1  Photo 2
Photo 2  Photo 3
Photo 3  Photo 4
Photo 4