Waterwall tubes in most of the fossil-fired boiler are made of carbon steel. In pure DM water, or in very dilute acid or alkaline solutions at boiler temperature, it normally corrodes very slowly to form the black iron oxide known as magnetite (Fe3O4). The overall reaction is:
3 Fe + 4 H2O ——-> Fe3O4 + 4 H2
The corrosion rate is dependent by the rate at which the reactant (water) can reach the metal surface and the reaction product can leave the surface. In nearly neutral solutions, magnetite is very slightly soluble and it deposits as coherent and tenacious surface film, which greatly impedes this two-way chemical traffic. The transport processes are dominated by slow state diffusion through the oxide layer and the corrosion rate is virtually independent of solution composition. The corrosion rate is also diminishes with time as the oxide thickness grows. Even after years of exposure, the layer is no more than a few microns thick.
In more alkaline or more acid solutions, magnetite becomes increasingly soluble and precipitates in a different physical form. Instead of yielding a strongly coherent film, it has a more porous structure. Soluble species can now diffuse relatively rapidly through the film and the corrosion rate is much faster, although it still falls off the time as the oxide accumulated.
In many countries there are mainly three types of Medical Laboratories as per the types of investigations carried out. 1. Clinical Pathology 2. Clinical Microbiology & 3. Clinical Biochemistry laboratories. 1. Clinical Pathology: Haematology, Histopathology, Cytology, Routine Pathology2. Clinical Microbiology: Bacteriology, Mycobacteriology, Virology, Mycology, Parasitology, Immunology, Serology. The deposition of salts and corrosion products observed in different waterwall tubes has shown in the photograph no.1-4.
Photograph no.1&2 showing waterwall tubes of 60 MW boilers having a very thick deposit ranging thickness 1.0-1.5 mm.
Photograph no.3 & 4 showing a waterwall tube of 200 & 110 MW boilers having a thick iron oxide as well as hardness salt deposition which is non uniform in nature.
IRC is regularly carrying out such studies to find out the reason of the failures and are also giving remedial actions to prevent it, if required we can also carry out chemical cleaning of boiler.
IRC is a service provider having expertise in ndt, residual life assessment, fitness for service, advanced ndt, failure investigation, chemical cleaning, certification of storage tanks as per chief controller of explosives guidelines , consultancy for boiler water chemistry and training
read more IRC Engineering Services
IRC Engineering Services
 Photo 1
Photo 1  Photo 2
Photo 2  Photo 3
Photo 3  Photo 4
Photo 4 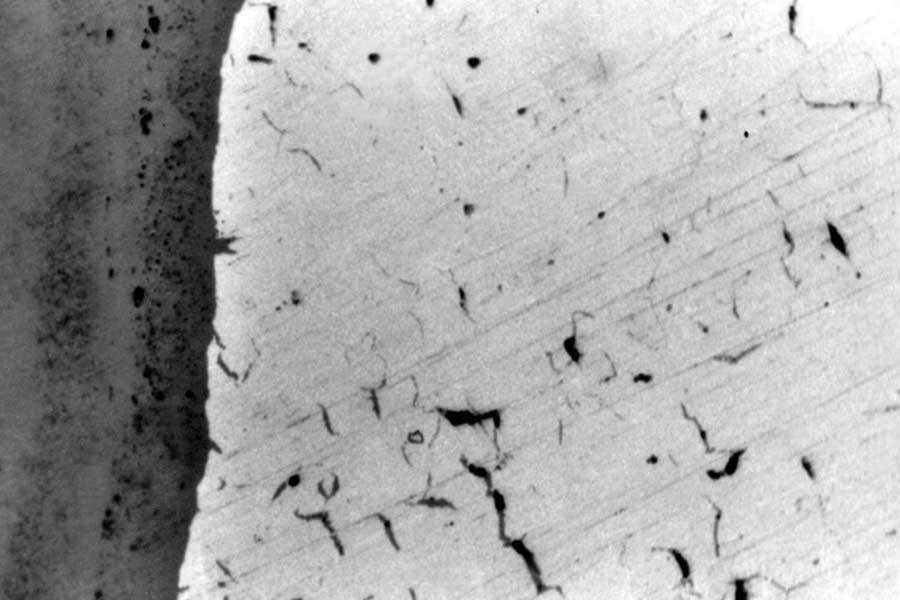







Recent Comments